Quality



SteriTek is an ISO 13485 certified, FDA registered, DEA registered, CBER Tissue Registered, MedAccred Certificates well as State of California Medical Device and Drug Manufacturing licensed facility.
To request a Quality Manual, please send an email to our Quality Department at quality@steri-tek.com.
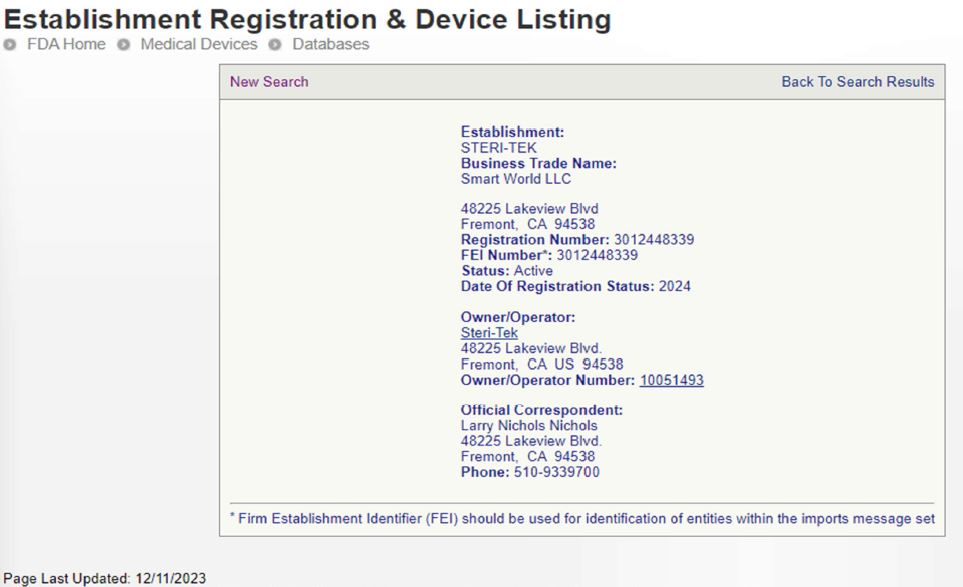
SteriTek is FDA Registered: (Owner Operator Number: 10051493)
Certifications
ISO Certification
International Irradiation Association
DEA Registration
FDA Registration
MedAccred Sterilization Accreditation
State of California Device Manufacturing License
State of California Drug Manufacturing License
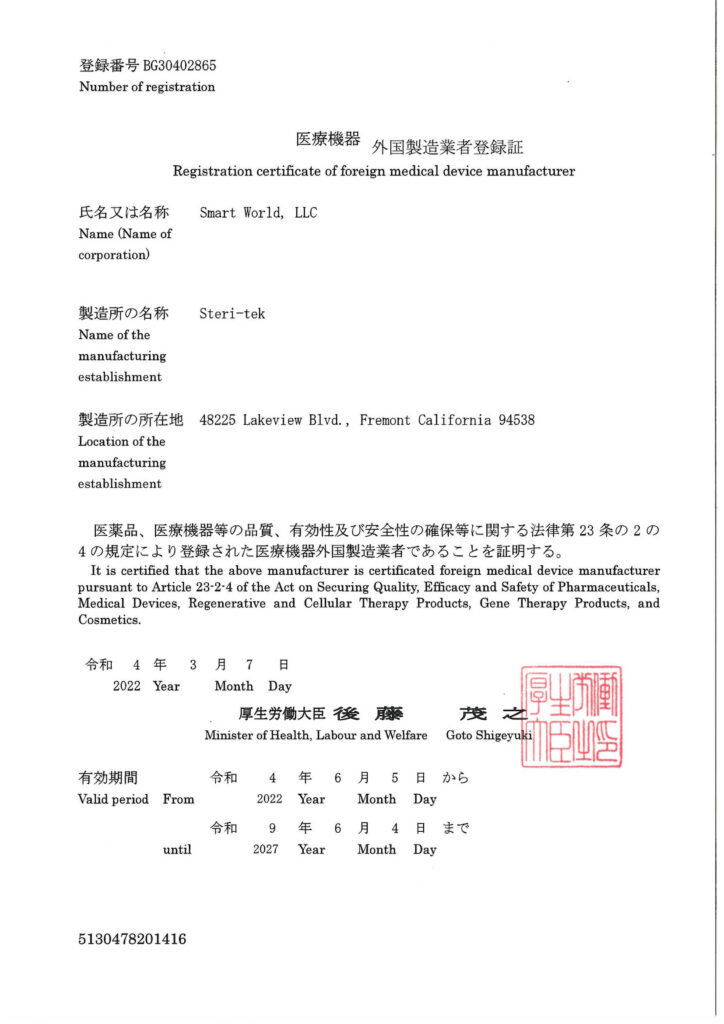
Japanese Ministry of Health Registration
Women/Minority Business Enterprise (WMBE) Certified